ASCO 2020: The Role of Neoadjuvant Therapy for Patients with Localized Prostate Cancer - UroToday
(UroToday.com) For patients with high risk localized prostate cancer, 15-year prostate cancer survival ranges from 20-38%. However, unlike other tumor types where neoadjuvant therapy has been shown to improve overall survival outcomes (breast, bladder, rectal), there remains a paucity of randomized data showing improved long-term outcomes with neoadjuvant therapy for prostate cancer. Current NCCN, AUA, and ASCO guidelines do not recommend neoadjuvant therapy outside of a clinical trial. Prior studies, such as CALGB 90203 (Randomized patients to radical prostatectomy or neoadjuvant ADT + Docetaxel x 6 cycles followed by prostatectomy) did not show improvement in 3-year PSA PFS (Eastham et al, AUA 2019).
More recently, neoadjuvant studies have been conducted with second-generation androgen antagonists such as abiraterone and enzalutamide, and this has demonstrated that 6 months of neoadjuvant ADT + enza can achieve pathologic complete response (CR) in some patients.1 Prior analysis by Rana R. McKay, MD has shown that a pathologic response can correlate with PSA recurrence.2

These two studies presented at this ASCO evaluate the safety and pathologic responses to intensive neoadjuvant hormonal therapy with combination apalutamide and leuprolide with or without abiraterone.
Abstract 5503: Results of a Phase II Trial of Intense Androgen Deprivation Therapy Prior to Radical Prostatectomy in Men with High-Risk Localized Prostate Cancer:
The first study (McKay et al) randomized patients with high-risk prostate cancer (Gleason ≥4+3, PSA >20, T3 disease, and lymph nodes <20 mm) to either abiraterone + prednisone + apalutamide + leuprolide (APAL) or abiraterone + prednisone + leuprolide (APL) for a total of 6 cycles (each cycle 28 days), followed by radical prostatectomy.
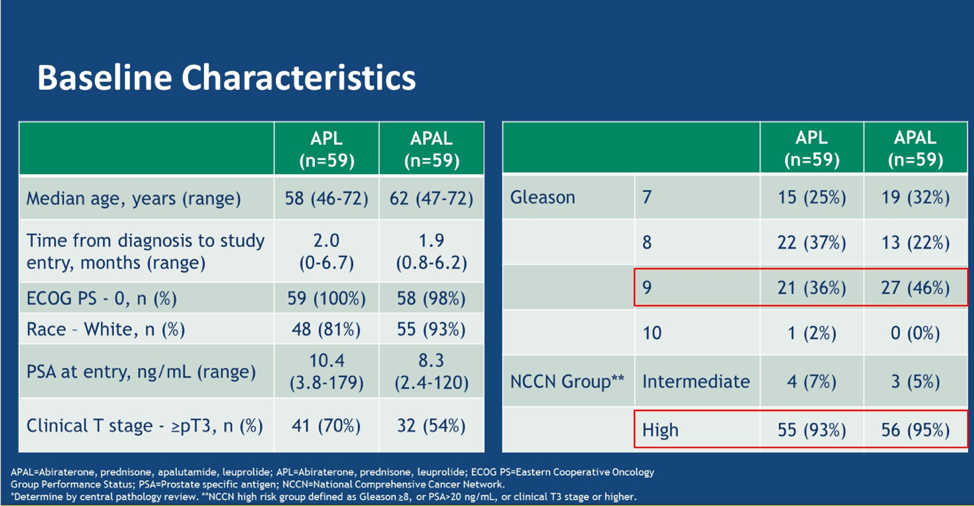
A total of 119 patients were enrolled, and 97% of patients completed the 6 cycles of therapy followed by radical prostatectomy. Baseline characteristics are shown below. This was a very high-risk population – 46% had Gleason 9/10 disease, and 59% fit in the NCCN high-risk category.
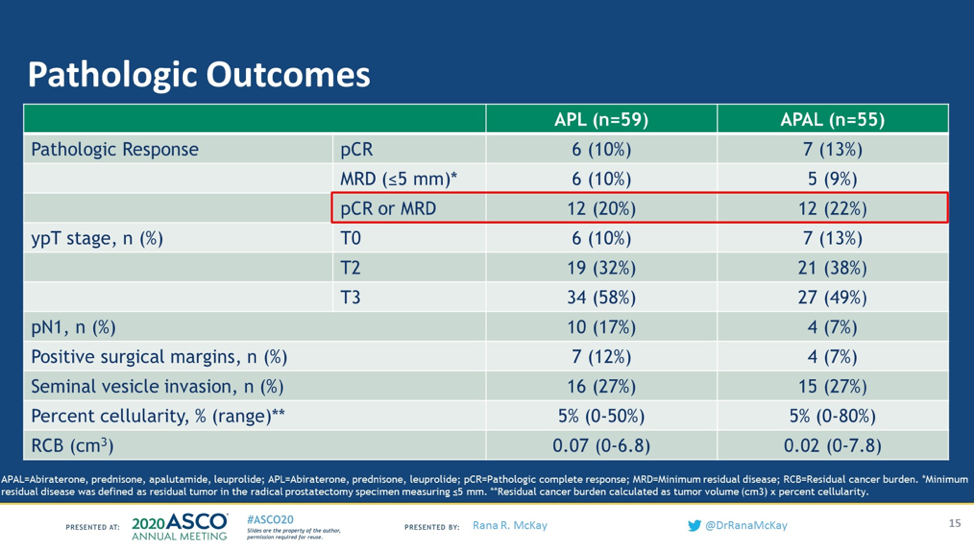
The primary endpoint of this study was the pathologic complete response rate or minimum residual disease (tumor <5mm). 13% of patients had a complete pathologic response to the APAL arm compared with 10% of patients on the APL arm. 9% of patients achieved MRD in the APAL arm compared with 10% of those on the APL arm. Given that almost all the patients achieved a dramatic PSA decline, PSA did not correlate with pathologic response.
In terms of safety, 11% of patients had had grade 3 treatment-related adverse events. The most common AEs were hypertension, elevated ALT, and elevated AST. There were no grade 4 or 5 adverse events
Abstract 5504: Neoadjuvant apalutamide (APA) plus leuprolide (LHRHa) with or without abiraterone (AA) in localized high-risk prostate cancer (LHRPC):
The second neoadjuvant study by Efstathiou et al also evaluated 6 months of APAL and APL in localized high risk (≥ cT2 + Gleason Score ≥ 8 or ≥ cT2b + Gleason ≥ 7 + PSA > 10 ng/mL) followed by radical prostatectomy.
63 of 65 patients enrolled had RP. 98% of the patients had a PSA <0.1 and 40% of patients had the organ-confined disease at the time of surgery. Prostatectomy specimens revealed significant heterogeneity in tumor viability despite uniform PSA responses. 16% of patients receiving APL had a complete response and 10% of patients receiving APAL had a complete response – this was not a significant difference, consistent with the prior study.

Tumor volume and tumor cellularity varied significantly. The finding of ≤ypT2N0 did not correlate with Gleason score, but did correlate with a pre-specified molecular signature, PTEN expression, and absence of cribriform/intraductal spread.
A 4-marker candidate signature (PTEN loss, nARV-7 presence, Glucocorticoid receptor (GR) high, or Ki67>10%) was predictive of resistance.
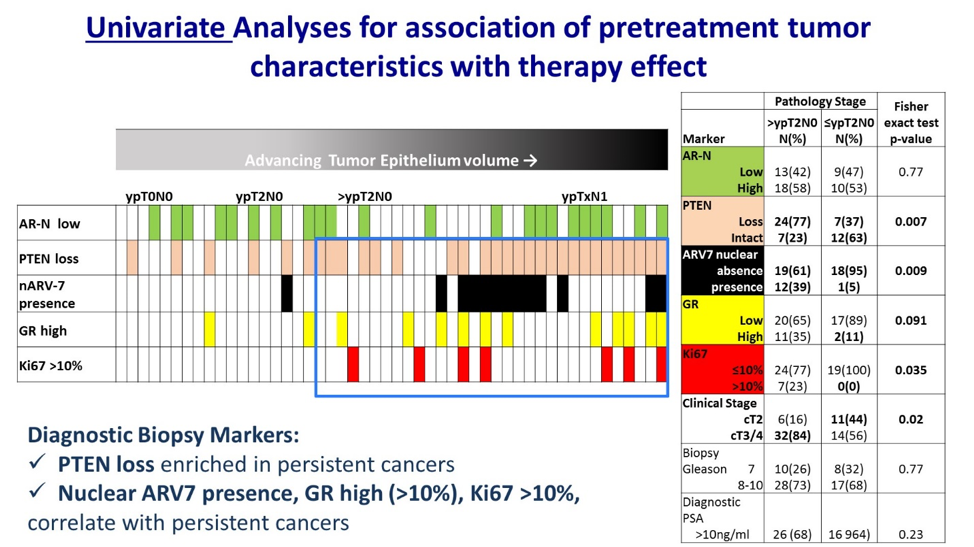
These two studies show that the role of neoadjuvant therapy for patients with localized prostate cancer has yet to be definitively defined but provides evidence that certain genomically selected subsets of patients may be good candidates for neoadjuvant therapy. Some biomarkers predict for resistance (PTEN, nARV7, GR>10%, Ki67>10%) which suggests either a different form of neoadjuvant therapy may be necessary, or perhaps these patients should be taken to surgery first followed by adjuvant therapy. There does not appear to be a one size fits all approach for neoadjuvant therapy in prostate cancer. Future work including genomics and transcriptomics in high-risk prostate cancer may be important to clearly define which patients most benefit from neoadjuvant therapy.3
Presented by: Rana R. McKay, MD, Medical Oncologist, Assistant Professor of Medicine, UC San Diego Health, San Diego, CA and Eleni Efstathiou, MD, Ph.D., Associate Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas
Written by: Jason Zhu, MD. Medical Oncologist, Division of Genitourinary Cancers, Levine Cancer Institute, Twitter: @TheRealJasonZhu, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
References:
- VanderWeele DJ, Turkbey B, Karzai F, et al. Neoadjuvant enzalutamide and androgen deprivation therapy for high-risk prostate cancer: Early results from a feasibility trial. American Society of Clinical Oncology; 2018.
- McKay RR, Montgomery B, Xie W, et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate cancer and prostatic diseases 2018;21:364-72.
- Beltran H, Wyatt AW, Chedgy EC, et al. Impact of therapy on genomics and transcriptomics in high-risk prostate cancer treated with neoadjuvant docetaxel and androgen deprivation therapy. Clinical Cancer Research 2017;23:6802-11.


Comments
Post a Comment