Stage 3 Cancer: Definition, Diagnosis, Treatment, Prognosis
TIL Therapy For Non-Small Cell Lung Cancer May Be Effective
There is evidence that an emerging type of immunotherapy called TIL therapy may be successful in treating more solid tumors.
Research published in Cancer Discovery shows this treatment works in some patients with non-small cell lung cancer. The lead researcher of the multicenter clinical trial was Memorial Sloan Kettering Cancer Center (MSK) lung cancer specialist Adam Schoenfeld, MD.
"The patients who came to us in this trial were very sick and had often exhausted all other options," says Dr. Schoenfeld, a thoracic oncologist, cellular therapist, and early drug development specialist. "The fact that 21% of the non-small cell lung cancer patients responded to TIL therapy is significant. Some patients remained free of cancer relapse for more than two years after the treatment."
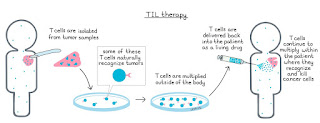
TIL Therapy Clinical Trial Results for Non-Small Cell Lung Cancer PatientsTIL (pronounced "till") stands for tumor-infiltrating lymphocytes, a specialized type of white blood cell. The approach involves removing TIL cells from the tumor, growing them into large numbers, and then putting them back into the patient. There, the TIL cells can seek out and destroy cancer cells anywhere in the body.
The phase 2 clinical trial published in Cancer Discovery involved 28 patients treated at several hospitals in the United States and Europe.
The scans of patients before and after the treatment are striking.
Dr. Adam Schoenfeld is focused on developing cell therapies like TIL therapy for lung cancer.
TIL therapy was recently approved to treat metastatic melanoma that has not responded to other treatments. That treatment, called lifileucel (Amtagvi™), became the first cell therapy approved by the Food and Drug Administration to treat a solid tumor.
"TIL therapy has been studied in melanoma for many years, but until very recently no one had ever shown the treatment could be effective for lung cancer," Dr. Schoenfeld says. "This study is significant because it was one of the first times anyone demonstrated that generating TIL cells from lung tumors was feasible and that these cells could generate anti-tumor activity."
He explains that since this trial was launched at MSK four years ago, other trials have shown that for patients earlier in the course of disease — those who have not developed resistance to checkpoint inhibitors — the response rates are about twice as high. Those findings were presented at the World Conference on Lung Cancer in September 2023.
How TIL Therapies WorkTIL therapies take advantage of the immune system's natural ability to recognize and attack cancer. They are in a class of treatments known as cell therapies — the patient's own cells fighting cancer. Cell therapies are often called "living drugs" because once in the body, they continue to circulate through the bloodstream and fight cancer.
This study is significant because it was one of the first times anyone demonstrated that generating TIL cells from lung tumors was feasible and that these cells could generate anti-tumor activity.
Adam J. Schoenfeld lung cancer specialistFor many people with blood cancer, other cell therapies called CAR T have successfully controlled the cancer. TIL is another form of cell therapies for treating solid tumors, which make up the majority of cancers. This is why oncologists are excited about the prospects of TIL therapy.
How TIL Therapy Is Given to Lung Cancer PatientsTIL therapy involves several steps:
TIL therapy has serious side effects, including low blood counts that make patients weak and susceptible to infections. However, the majority of side effects are caused by the chemotherapy and the interleukin-2, not by the TIL cells. Interleukin-2 can also cause severe side effects, such as shortness of breath, heart problems, and kidney injury, if not monitored closely in the hospital.
To make TIL treatment safer, next-generation therapies are being developed that allow patients to avoid treatment with interleukin-2. For example, other potentially safer and more effective interleukins such as interleukin-15 may be engineered into the patients' TIL cells while they are in the lab, limiting their effects only to the cancer and not the rest of the body.
TIL Therapy for Lung Cancer Clinical Trials at MSKDr. Schoenfeld is leading a phase 2 trial for one of these next-generation TIL therapies, called OBX-115, in lung cancer. OBX-115 is also being studied for melanoma at several cancer centers, including at MSK, led by melanoma oncologist and cellular therapist Alexander Shoushtari, MD. Notably, MSK is currently the only hospital offering it for lung cancer.
Dr. Schoenfeld is also looking for other ways to make the therapy less difficult for patients. Some research has suggested that it may be possible to lower the doses of chemotherapy given before the infusion of the TIL cells, further reducing side effects of the treatment.
"TIL therapy has the potential to benefit so many people with lung cancer," Dr. Schoenfeld says. "This is only the beginning. As we can find ways to make it safer, TIL and other cell therapies will offer an important new therapy for a group of patients who have had little success with other treatments."
The lifileucel clinical trial was funded by Iovance Biotherapeutics, the company that makes the drug.
Dr. Schoenfeld reports a consulting/advisory role and participation in a Data Safety Monitoring Board or Advisory Board for Johnson & Johnson, KSQ Therapeutics, Bristol Myers Squibb (BMS), Merck, Enara Bio, Perceptive Advisors, Oppenheimer and Co, Umoja Biopharma, Legend Biotech, Iovance Biotherapeutics, Lyell Immunopharma, Prelude Therapeutics, Immunocore, Amgen, and Heat Biologics. He has received institutional research funding from GSK, PACT Pharma, Iovance Biotherapeutics, Achilles Therapeutics, Merck, BMS, Harpoon Therapeutics, and Amgen.
Sylvester Cancer Adding Cellular Therapy To Its Arsenal Against Metastatic Melanoma
image:
"Cell therapy has now become a viable option for patients with advanced melanoma," said Jose Lutzky, M.D., a skin cancer physician and director of cutaneous oncology at Sylvester Comprehensive Cancer Center. "The trial results represent a major advancement for our patients."
view moreCredit: Photo by Sylvester
MIAMI, FLORIDA (May 1, 2024) – Patients in South Florida with metastatic melanoma will soon have access to the first cellular therapy for this advanced form of skin cancer, following its recent approval by the Food and Drug Administration (FDA). The therapy, known as tumor-infiltrating lymphocyte therapy, or TIL, uses patients' own immune cells to battle their cancer. It will be available to patients at Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine as South Florida's only center offering this treatment.
According to a Centers for Disease Control and Prevention (CDC) 2020 report, Florida has the second highest number of new melanoma cases per year in the U.S.
Additionally, Sylvester researchers will be part of an upcoming clinical trial led by TIL's manufacturer, Iovance Biotherapeutics, to determine if certain modifications to the therapy can improve its effectiveness for even more patients.
Trials testing the TIL therapy, called lifileucel, before FDA approval showed a response rate of 32% among 153 patients. Lifileucel is approved for patients whose melanoma has progressed despite treatment with other forms of immunotherapy, including those involving checkpoint inhibitors, and targeted therapy for melanomas with a common mutation in the BRAF gene.
"Cellular therapy represents a major breakthrough in the battle against advanced melanoma," said Jose Lutzky, M.D., skin-cancer specialist and director of Cutaneous Oncology Services at Sylvester. "The trial results offer hope to our patients with this potentially deadly disease."
He added that in the clinical trial that led to lifileucel's approval, more than half of patients who responded favorably to the therapy had maintained that response for over three years.
Although TIL therapy has been studied in labs and clinical trials for decades, lifileucel represents the first FDA approval for any TIL treatment.
Unleashing the Immune Response
Tumor-infiltrating lymphocytes are naturally occurring immune cells composed of a mix of white blood cells, T cells and B cells that invade tumors to try to fight them. To boost their natural cancer-killing abilities, TIL therapy involves surgically removing a patient's tumor, isolating the lymphocytes within it and then growing them in larger quantities at a special manufacturing facility before reinfusing them into the patient.
Unlike other types of cell therapy, such as CAR-T cell therapy, the lymphocytes are not genetically engineered to recognize cancer because they are already attuned to the patient's specific tumor. The TIL method also involves treatments after infusion to activate and stimulate the lymphocytes within the patient's body.
Lutzky said the therapy, which typically includes surgery, high doses of chemotherapy before infusion and subsequent treatments can take its toll on patients, and those with heart conditions or other health problems may be unable to tolerate the treatment.
"These are not easy treatments, but many patients do have great responses," he explained.
Upcoming Clinical Trial
Lutzky plans to begin enrollment for Sylvester's participation in the phase 2 Iovance trial soon and hopes to enroll 10 or more patients in the next year. Eligible participants will be patients with advanced melanoma whose previous treatments failed against the disease.
The trial will test a variation of TIL therapy in which immune cells are genetically engineered to remove a gene called PD-1, which acts as a natural block on immune cell activity. Cancerous cells often hijack this process to switch off the body's immune cells.
"Removing PD-1 from lymphocytes may enhance their cancer-killing ability in the body," Lutzky said. "Laboratory studies and an early-stage clinical trial of these modified cells showed that they are just as active as the non-engineered lymphocytes."
The Journey to Treatment Breakthroughs
Before joining Sylvester, Lutzky participated in clinical trials that led to FDA approval of another immunotherapy for metastatic melanoma -- a checkpoint inhibitor called ipilimumab. In fact, melanoma was the first cancer for which checkpoint inhibitors were approved as a treatment.
Because melanoma response is closely tied to the immune system, immunotherapies tend to work better with this cancer than other types. Moreover, lessons learned from treating melanoma with immunotherapy have enabled researchers and clinicians to apply those findings to fight other cancers, Lutzky said.
"Melanoma was the first tumor where checkpoint inhibitors were found to be effective and established what we now call the fourth pillar of cancer treatment, adding immunotherapy to surgery, chemotherapy and radiation," he said. "It's been a really amazing journey."
Read more on the InventUM blog and follow @SylvesterCancer on X for the latest on its research and care.
# # #
Disclaimer: AAAS and EurekAlert! Are not responsible for the accuracy of news releases posted to EurekAlert! By contributing institutions or for the use of any information through the EurekAlert system.
Ludwig Lausanne Scientists Identify And Show How To Target A Key Tumor Defense Against Immune Attack
image:
Matteo Morotti, Ludwig Lausanne
view moreCredit: Ludwig Cancer Research
April 24, 2024, NEW YORK – A Ludwig Cancer Research study has discovered how a lipid molecule found at high levels within tumors undermines the anti-cancer immune response and compromises a recently approved immunotherapy known as adoptive cell therapy (ACT) using tumor infiltrating lymphocytes, or TIL-ACT. In this individualized cell therapy, TILs—CD8+ T cells that kill cancer cells—are expanded in culture from a patient's tumor samples and reinfused into the patient as a treatment.
Researchers led by Ludwig Lausanne's Matteo Morotti, Alizee Grimm, Denarda Dangaj Laniti and Director George Coukos report in the current issue of Nature their discovery—based on analyses of data and samples collected in TIL-ACT clinical trials—of the mechanism by which the lipid, prostaglandin E2 (PGE2), poisons TIL metabolism to serve as a checkpoint against their anti-tumor activity. They also show that disrupting that mechanism during the cell culture processes employed in TIL-ACT dramatically improves the therapeutic potential of transferred TILs in mouse models of cancer.
A companion study led by researchers at the Technical University of Munich, done using mouse models of cancer, confirms the Ludwig Lausanne team's findings and appears in the same issue of Nature. Several researchers share authorship of both papers.
"These findings have important implications for cancer immunotherapy in general, as they identify a novel and eminently targetable checkpoint against the function of infiltrating cytotoxic T cells in the tumor microenvironment," said Coukos. "More immediately, we can now complete the circle here at Lausanne: we have harnessed the data and materials obtained from TIL-ACT trials in humans to advance our understanding of tumor immunology and can now apply what we have learned to improve outcomes for patients undergoing such therapies. This is a key element of our strategy—and of Ludwig's overall mission."
The Ludwig Lausanne researchers began by examining gene expression data collected from T cells isolated from patients for TIL-ACT. Their analysis revealed that the TILs that already bore markers of response to interleukin-2 (IL-2)—a factor essential to functional vitality and proliferation of T cells—were the ones that expanded best in culture. The researchers also tracked the growth in culture of 215 individual tumor-reactive TILs that respond to IL-2, which is a key component of TIL cultures, and showed that this is indeed the case.
Further examination of gene expression data collected from clinical trial samples revealed that TILs that responded poorly to IL-2 expressed relatively large numbers of receptors for PGE2 on their surfaces. In line with that finding, anti-cancer TILs from tumor fragments that had high levels of PGE2 were found to expand poorly in culture.
To explore how PGE2 compromises TIL-ACT, the researchers began by identifying a signature of gene expression associated with PGE2's effects on T cells.
"We found that patients' tumor-reactive TILs that bore this PGE2 signature expanded very poorly in culture, and that these TILs were often in states that would otherwise be essential to therapeutic responses," said Morotti. "Further, corresponding clinical trial data revealed that the melanoma patients whose TILs bore that signature had responded poorly to TIL-ACT therapy."
The researchers next probed how PGE2 exerts its effects on TILs. They found that the lipid, acting through its receptors (EP2 and EP4), disrupts the ability of T cells to sense and respond to IL-2 by monkeywrenching the assembly of the IL-2 receptor's component proteins. The resulting loss of IL-2 stimulation initiates a cascade of biochemical events in the cells that culminates in profound metabolic dysfunction, inducing a functional lethargy in the TILs known as "anergy" and ultimately triggering ferroptosis, a type of programmed cell death.
The researchers also examined whether blocking PGE2 could overcome these effects.
"By adding a drug that inhibits PGE2 production to the TIL culture medium we restored the ability of TILs to respond to IL-2 and improved the expansion of tumor-reactive TILs more than two-fold," said Grimm. "Small molecules that block PGE2 interaction with its receptors had a similar effect. These studies not only confirmed our hypothesis but also identify a means to swiftly translate our findings for clinical application."
The researchers show that, grown in the presence of PGE2 inhibitor, tumor-reactive TILs become responsive to IL-2 once again and show signs of renewed metabolic fitness and functional vitality. These TILs prevented cancer cells taken from the tumors they previously inhabited from forming new tumors in mice, while the same TILs grown sans PGE2-inhibitor did not. The revitalized TILs were also better agents of TIL-ACT therapy in a mouse model.
The companion study in Nature, led by the Jan Böttcher lab in Munich, confirms the findings of this study in mouse models of cancer. It also shows that the inhibition of IL-2 signaling in TILs occurs within the microenvironment of tumors. Engagement of the PD-1 checkpoint on T cells, which is currently targeted by several immunotherapies, is by contrast thought to occur mainly in the lymph nodes around tumors.
"When we learned we were working on essentially the same problem, we decided to combine our efforts and publish our shared discoveries as back-to-back publications in the same journal," said Dangaj Laniti. "That spirit of cooperation, rather than the usual, fierce competition, has borne fruit in the discovery of a previously unknown immune checkpoint in tumors that is mechanistically distinct from the PD-1 checkpoint that cancers exploit to foil anti-tumor T cells. It has been very gratifying to be a part of this partnership, whose discoveries will surely be of great benefit to cancer patients."
In addition to their posts at Ludwig Lausanne, George Coukos directs the Department of Oncology at the Lausanne University Hospital (UNIL CHUV) and codirects the Swiss Cancer Center Léman and Denarda Dangaj Laniti is the Head of the Tumor Microenvironment and Biomarker Discovery Lab at Ludwig Lausanne.
This study was supported by Ludwig Cancer Research, Swiss Cancer League Foundation, the German Research Foundation and grants from the Biltema Foundation, Paul Matson Foundation and Cancera Foundation.
# # #
About Ludwig Cancer Research
Ludwig Cancer Research is an international collaborative network of acclaimed scientists that has pioneered cancer research and landmark discovery for more than 50 years. Ludwig combines basic science with the translation and clinical evaluation of its discoveries to accelerate the development of new cancer diagnostics, therapies and prevention strategies. Since 1971, Ludwig has invested nearly $3 billion in life-changing science through the not-for-profit Ludwig Institute for Cancer Research and the six U.S.-based Ludwig Centers. To learn more, visit www.Ludwigcancerresearch.Org.
For additional information please contact communications@ludwigcancerresearch.Org.
Disclaimer: AAAS and EurekAlert! Are not responsible for the accuracy of news releases posted to EurekAlert! By contributing institutions or for the use of any information through the EurekAlert system.


Comments
Post a Comment