APCCC 2019: Oligometastatic Prostate Cancer – Definitions and Concepts - UroToday
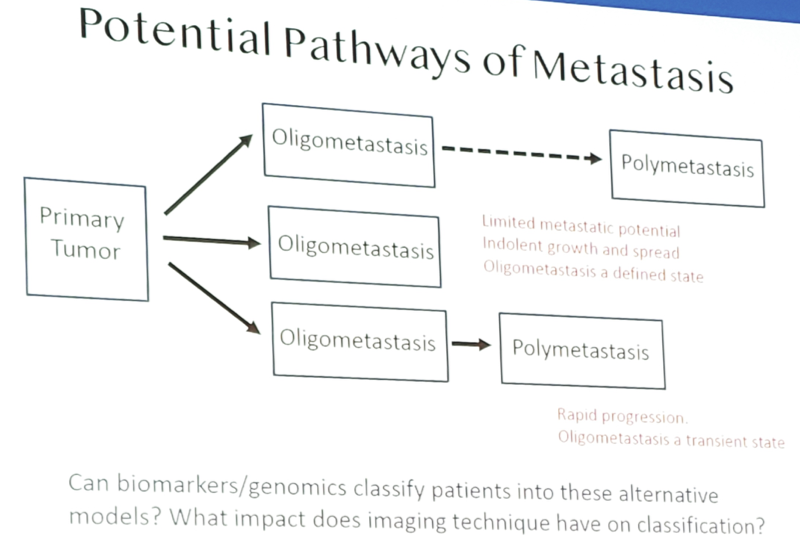
Oligometastasis is hypothesized to represent a state in which a tumor lacks all the necessary “hallmarks of cancer” to metastasize to specific sites, grow rapidly, or metastasize secondarily. However, there is no genomic data to define or classify the oligometastatic state as of yet, although the GAP6 initiative is exploring these pathways.
Dr. Reiter notes that there are several definitions for oligometastasis, including:
- Site of disease – bone only, any site (bone, node, and/or soft tissue), bone and other sites (node and/or soft tissue)
- Number of lesions – 1-5, in general
- Temporal pattern – synchronous (primary in place), metachronous (primary previously treated), progressive (induced by prior systemic therapy)
- Castration status – hormone sensitive (most common), castration-resistant
There has also been a big impact of imaging on defining the metastatic state. Most published studies are based on conventional imaging (MRI, CT, and bone scan), and first generation molecular imaging modalities (NaF, choline, acetate). Without question, the definition and diagnosis of oligometastasis will depend on the staging modality, as a significant reclassification (upstaging) occurs with more contemporary molecular imaging (ie. PSMA, axumin), such as non-metastatic to oligometastatic and oligometastatic to polymetastatic. Indeed, the extrapolation of clinical data obtained with conventional imaging to that obtained with PSMA may not be warranted. Dr. Reiter also cautions that even the best molecular imaging tool will miss (understage) a significant percentage of metastases (<5 mm, low PSMA). In high risk patients, Ga-PSMA has a 30-40% sensitivity on a per patient basis for nodes, and a 24% sensitivity on a per node basis1.
Dr. Reiter notes that there are two important clinical questions relevant to clinical trials in oligometastatic disease: (i) For synchronous disease, does “local” treatment of all visualized disease impact patient or disease related outcomes? (ii) For metachronous or progressive disease, does metastasis directed therapy impact patient or disease related outcomes? Furthermore, there are issues surrounding valid endpoints: Survival? Time to polymetastatic progression? Time to systemic therapy?
Dr. Reiter concluded with several take-home messages from his oligometastatic disease presentation:
- There is no clear consensus on oligometastatic disease as an existence as a discrete entity or its prevalence
- We don’t know if oligometastatic disease is a measure of indolent versus aggressive disease, clonal or polyclonal disease, etc. Genomic and other classifiers are needed
- There is no consensus on the role of imaging to define or manage oligometastatic disease, and trials must consider inclusion of PSMA imaging
- There is no consensus on appropriate clinical trial endpoints
Presented by: Robert E. Reiter, MD, UCLA Center for Health Sciences, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 - 31, 2019 in Basel, Switzerland
https://ift.tt/2Zvma44


Comments
Post a Comment